Curriculum
SPECIALIST IN MICROBIOLOGY
MICROBIOLOGY LABORATORY PROCEDURES
0/4LAB. IDENTIFICATION OF SIGNIFICANT ISOLATES
0/14-
STAPHYLOCOCCACEAE
-
STREPTOCOCCACEAE AND OTHER RELATED ORGANISMS
-
ENTEROBACTERIACEAE
-
NEISSERIACEAE AND MORAXELLACEAE
-
HAEMOPHILUS AND OTHER FASTIDIOUS GRAM-NEGATIVE SPECIES
-
OTHER FASTIDIOUS GRAM-NEGATIVE BACILLI, BORDETELLA AND LEGIONELLA SPP.
-
VIBRIO, AEROMONAS, PLESIOMONAS, AND CAMPYLOBACTER SPECIES
-
NONFERMENTING GRAM-NEGATIVE BACILLI
-
ANAEROBES OF CLINICAL IMPORTANCE
-
THE SPIROCHETES
-
CHLAMYDIA, RICKETTSIA, AND SIMILAR ORGANISMS
-
MYCOPLASMA AND UREAPLASMA
-
MTB AND NTM LESSON 1
-
MTB AND NTM LESSON 2 - LABORATORY DIAGNOSIS
MEDICALLY SIGNIFICANT FUNGI
0/3DIAGNOSTIC PARASITOLOGY
0/5MTB AND NTM LESSON 1
MYCOBACTERIUM TUBERCULOSIS AND NONTUBERCULOUS MYCOBACTERIA
INTRODUCTION
The most familiar species in the genus Mycobacterium are MTB and Mycobacterium leprae, the causative agents of tuberculosis (TB) and Hansen disease (leprosy), respectively. Both diseases have long been associated with chronic illness and social stigma.
General Characteristics
Mycobacteria are slender, slightly curved or straight, rod-shaped organisms. They are nonmotile and do not form spores. The cell wall has extremely high lipid content; thus, mycobacterial cells resist staining with Gram stain, at RT. Mycobacteria take up dye with increased staining time or application of heat but resist decolorization with acid-ethanol. This characteristic is referred to as “acid fastness”— hence, the term AFB.
Mycobacteria are strictly aerobic, but increased CO2 will enhance the growth of some species. Most pathogenic mycobacteria require 2 to 6 weeks of incubation on complex media at specific optimal temperatures. The rapidly growing species generally grow on simple media in 2 to 3 days at temperatures of 20°C to 40°C. M. leprae, fails to grow in vitro.
Clinical Significance of the MTB Complex
The MTB complex consists of M. tuberculosis, M. bovis (including the vaccination strain Bacillus Calmette-Guérin), M. africanum, M. canettii, and M. microti. M. africanum has been associated with TB cases in tropical Africa.
1. Mycobacterium tuberculosis
· Primary Tuberculosis
TB is usually a disease of the respiratory tract. Tubercle bacilli are acquired from persons with active disease who are excreting viable bacilli by sneezing or talking.
Airborne droplets containing bacteria, 1 to 5 µm in size, enter the respiratory tract of an exposed individual and reach the lung alveoli.
M. tuberculosis cells are phagocytized by alveolar macrophages and are capable of intracellular multiplication.
In a person with adequate cellular immunity, the macrophages are able to destroy the intracellular mycobacteria. There is then a regression and healing of the primary lesion and any disseminated foci of infection.
In case of little infection and a strong hypersensitivity reaction to mycobacterial antigen induces the formation of hard tubercle or granuloma of lymphocytes, macrophages, fibroblasts, and capillaries followed by healing fibrosis, encapsulation, and calcification, with scar formation.
If the antigen load and hypersensitivity reaction are both high, tissue necrosis from the enzymes of degenerating macrophages can occur; the tissue response is less organized, and no granuloma is formed.
Without granuloma or necrosis, lesions may heal without obvious pathology. With necrosis, a caseous material may be present. After healing of first-degree infection, the bacilli are not totally eradicated but can remain viable in granulomas for months or years a potential for reactivation of TB.
In primary TB children may demonstrate a nonproductive cough and fever, with or without shortness of breath; these symptoms are unusual in adults.
· Reactivation Tuberculosis
Reactivation TB occurs when there is an alteration or suppression of the cellular immunity in the infected host that favors replication of the bacilli and progression to disease.
The symptoms are slow in developing (insidious) and consist of fever, shortness of breath, night sweats and chills, fatigue, anorexia, and weight loss. Some individuals may have cough, chest pain, and productive sputum.
Hemoptysis, indicating cavitation and necrosis, occurs in 25% of cases.
In chronic disease, fibrosis, loss of lung volume, and calcifications will be demonstrated.
The PPD skin test may be negative in up to 25% of these cases; diagnosis is confirmed by stained smear and culture of sputum, gastric aspirates, or bronchoscopy specimens.
If diagnosis and treatment are delayed there may be complications including empyema, pleural fibrosis, massive hemoptysis and to lesser extent adrenal insufficiency, and hypercalcemia.
In patients with AIDS and TB with drug-resistant bacilli, the risk of progression to disease from infection is quite high.
The diagnosis is usually made by stained smears and culture, with a rate of sensitivity similar to that in the non-AIDS patient.
· Extrapulmonary Tuberculosis
Extrapulmonary TB occurred much less commonly than pulmonary TB, and commonly seen in individuals with HIV infection or pulmonary disease as well as in children TB cases.
Miliary TB refers to the seeding of many organs through hematogenous spread. The most common sites of spread of M. tuberculosis are the spleen, liver, lungs, bone marrow, kidney, adrenal gland, and eyes. Other forms of extrapulmonary TB include pleural, lymphadenitis, GIT, skeletal, meningeal, peritoneal, and genitourinary infections.
Almost any organ of the body can be infected by M. tuberculosis; in addition to peritonitis TB, cutaneous TB, laryngitis, otitis, adrenal glands and eyes, and breast infections.
Up to 70% of HIV-infected patients may have extrapulmonary TB alone or, usually, in combination with pulmonary disease; lymph nodes (especially mediastinal), genitourinary tract, and the abdominal cavity are mostly affected. Bacteremia is not uncommon.
· Identification of Mycobacterium tuberculosis
Colonies of this slowly growing species are typically raised, with a dry, rough appearance. The colonies are nonpigmented and classically described as being buff-colored. Optimal growth occurs at 35°C to 37°C.
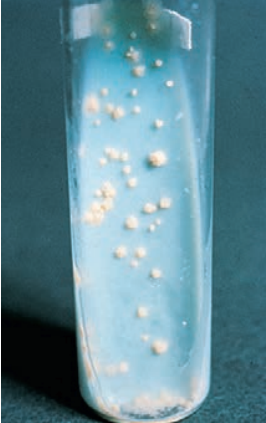 Mycobacterium tuberculosis growing on Löwenstein-Jensen medium.
Mycobacterium tuberculosis growing on Löwenstein-Jensen medium.
Biochemically, M. tuberculosis is positive for niacin accumulation, reduction of nitrate to nitrite, and production of catalase, which is destroyed after heating (heat-stable, catalase-negative).
Isoniazid-resistant strains may not produce catalase at all.
M. tuberculosis is inhibited by nitroimidazopyran or p-nitroacetylamino-β-propiophenone (NAP).
This species can be distinguished from M. bovis by the inhibition of M. bovis by thiophene-2-carboxylic acid hydrazide (T2H) and pyrazinamidase activity.
· Treatment
The treatment of TB involves the use of more than one antimycobacterial agent.
For pulmonary TB, treatment involves a 9-month course of therapy with isoniazid and rifampin, usually once per day the first month and twice a week thereafter.
Many regimens also include a 2- to 8-week initial course of streptomycin or ethambutol. Most individuals clear their sputum of AFB within the first 2 months.
Pyrazinamide (PZA) may be added along with isoniazid and rifampin to boost bactericidal levels intracellularly in macrophages.
· Multidrug-Resistant Mycobacterium tuberculosis
Risk factors for MDR-TB may include previous treatment for TB, residence in an area endemic for drug resistance, or close contact with an individual who is infected with MDR-TB.
Drug resistance is usually acquired by spontaneous mutations as a result of the lack of patient compliance. MDR-TB is a resistance to at least isoniazid and rifampin (first-line).
Extensively drug-resistant TB (XDR-TB) is a resistance to isoniazid and rifampin plus resistance to any fluoroquinolone and at least one of three injectable second-line anti-TB drugs—the aminoglycosides amikacin, kanamycin, or capreomycin.
Because of the threat of MDR-TB and XDR-TB, it is important for laboratories to identify Mycobacterium spp. rapidly and perform antimicrobial susceptibility testing so that appropriate therapy can be administered as quickly as possible.
For cases of resistance to isoniazid or rifampin, second-line antituberculosis drugs may include aminoglycosides and fluoroquinolones for extended period.
2. Mycobacterium bovis
Mycobacterium bovis produces TB primarily in cattle but also in humans.
The disease in humans closely resembles that caused by M. tuberculosis and is treated similarly.
It grows very slowly on egg-based media, producing small, granular, rounded, nonpigmented colonies with irregular margins after 21 days of incubation at 37°C.
On Middlebrook 7H10 medium, colonies are similar to those of M. tuberculosis but slower to mature. Most strains of M. bovis are niacin-negative, do not reduce nitrate, and do not grow in the presence of T2H, that distinguish the species from most strains of M. tuberculosis.
3. Clinical Significance of Nontuberculous Mycobacteria
Most NTM are found in soil and water. They are implicated as opportunistic pathogens in patients with underlying lung disease, immunosuppression and AIDS, or percutaneous trauma.
Chronic pulmonary disease resembling TB is the usual clinical presentation, although a few species are more often associated with cutaneous infections.
Infections caused by the NTM are not considered transmissible from person to person.
SLOWLY GROWING SPECIES
1. Mycobacterium avium Complex (MAC)
Mycobacterium avium and M. intracellulare are part of the Mycobacterium avium complex (MAC).
Clinical Infections. Pulmonary disease resulting from MAC infection is similar to that of TB – cough, fatigue, weight loss, low-grade fever, and night sweats.
Disseminated disease is common in immunocompromised or hematologic abnormalities patients. MAC infections are the most common systemic bacterial infection in patients with AIDS. The loss of CD4+ T cells reduces the activation of the macrophage to kill MAC organisms.
The clinical outcome of MAC lung disease is unpredictable, so the management of affected patients can be difficult.
Therapy for underlying pulmonary disease (e.g., bronchodilators, broad-spectrum antimicrobials, smoking cessation), and periodic sputum cultures may be all that is required for most patients.
For patients with significant symptoms multidrug therapy is indicated.
For children with cervical lymphadenitis from MAC, excisional surgery without chemotherapy is usually successful.
A combination of surgical excision and chemotherapy is the usual treatment for adults with localized nonpulmonary disease.
Most cases of respond to multidrug regimens. Multidrug therapy consisting of ethambutol, rifampin (or rifabutin), clofazimine, and an aminoglycoside has become successful for disseminated disease in immunosuppressed patients without AIDS as well clinical improvement in most (but not all) patients with AIDS.
Laboratory Diagnosis. Because the two species in the MAC are so similar, most Lab do not distinguish between them but report isolates of both species as MAC.
On primary isolation media, these organisms grow slowly, producing thin, transparent or opaque, homogeneous smooth colonies. A small proportion of strains may exhibit rough colonies. The colonies are nonpigmented, but they may become yellow with age. Optimal growth to is 37°C.
Microscopically, the cells are short, coccobacillary, and uniformly stained, without beading or banding. Long, thin, beaded bacilli resembling Nocardia spp. may be seen in stains of very young cultures.
Production of a heat-stable catalase and the ability to grow on media containing 2 µg/ mL of T2H help for identification of MAC.
2. Mycobacterium avium subsp. paratuberculosis
This subspecies is the causative agent of Johne disease, an intestinal infection occurring as a chronic diarrhea in cattle, sheep, goats, and other ruminants.
M. avium subsp. paratuberculosis is difficult to cultivate because of its very slow growth rate (3 to 4 months) and its need for a mycobactin-supplemented medium for primary isolation.
Mycobactin is an iron-binding hydroxamate compound produced by other mycobacterial species.
3. Mycobacterium kansasii
Mycobacterium kansasii is second to MAC as the cause of NTM lung disease. As with other NTM, infections are not normally considered contagious from person to person.
Clinical Infections. The most common manifestation is chronic pulmonary disease involving the upper lobes, usually with evidence of cavitation and scarring.
Extrapulmonary infections, include lymphadenitis, skin and soft tissue infections, and joint infection, have been reported occasionally.
Disseminated M. kansasii infection has been reported in severely immunocompromised patients, particularly those with AIDS.
When MTB AST is used, most strains of M. kansasii are susceptible to rifampin and ethambutol, partially resistant to isoniazid and streptomycin, and resistant to pyrazinamide.
For treatment of pulmonary disease caused by M. kansasii, a multidrug regimen of isoniazid, rifampin, and ethambutol is currently recommended.
· Laboratory Diagnosis.
M. kansasii is a slow-growing organism that appears as long rods with distinct crossbanding.
M. kansasii grows at 37°C, and colonies appear smooth to rough, with wavy edges and dark centers when grown on Middlebrook 7H10 agar.
Colonies are photochromogenic (they form a pigment when exposed to light) but are nonpigmented in the dark.
Scotochromogenic (produce pigment in light and dark) and nonchromogenic strains are rarely isolated.

M. kansasii growing on Löwenstein-Jensen medium showing photoreactivity. Left, Before exposure to light. Right, After exposure to light.
With prolonged exposure to light, most strains form dark red crystals of β-carotene on the surface of and inside the colony. Most strains are strongly catalase-positive.
· Key characteristics are: growth rate similar to that of MTB at 37°C, strong photochromogenic properties, ability to hydrolyze Tween 80 in 3 days, strong nitrate reduction, catalase production, and pyrazinamidase production.
4. Mycobacterium genavense
Mycobacterium genavense has been reported as a cause of disseminated infections in patients with AIDS. It has also been associated with enteritis and genital and soft tissue infections in HIV-positive and HIV-negative immunocompromised patients.
This slow-growing, fastidious mycobacterium has been recovered in BACTEC culture systems but failed to grow on subculture to routine solid media.
Growth was obtained when Middlebrook 7H11 agar was supplemented with mycobactin.
Isolates yield positive test results for semiquantitative and heat-stable catalase, pyrazinamidase, and urease.
5. Mycobacterium haemophilum
Infections associated with M. haemophilum occur primarily in immunocompromised patients with Hodgkin disease and AIDS.
Submandibular lymphadenitis, subcutaneous nodules, painful swellings, ulcers progressing to abscesses, and draining fistulas are often the clinical manifestations. A unique characteristic of this organism is its requirement for hemoglobin or hemin for growth.
Isolation is accomplished on supplemented media, such as chocolate (CHOC) agar, Mueller-Hinton agar with 5% Fildes enrichment, and Löwenstein-Jensen (LJ) medium containing 2% ferric ammonium citrate.
Successful isolation on Middlebrook 7H10 agar with an X factor disk planted in the inoculated area has been reported. Optimal growth temperature is 28°C to 32°C; little or no growth occurs at 37°C. Colonies are rough to smooth and nonpigmented.
Microscopically, the cells are strongly acid-fast, short, occasionally curved bacilli without banding or beading, and arranged in tight clusters or cords.
6. Mycobacterium malmoense
Chronic pulmonary disease and cervical lymphadenitis are associated with the M. malmoese.
In Lab AST, M. malmoense is resistant to isoniazid, streptomycin, p-aminosalicylic acid, and rifampin and susceptible to ethambutol and cycloserine.
M. malmoense appears as a short coccobacillus without cross bands on acid-fast–stained smears. Colonies are smooth, glistening, and opaque, with dense centers. Color is non-pigmented to buff; exposure to light does not induce pigment production.
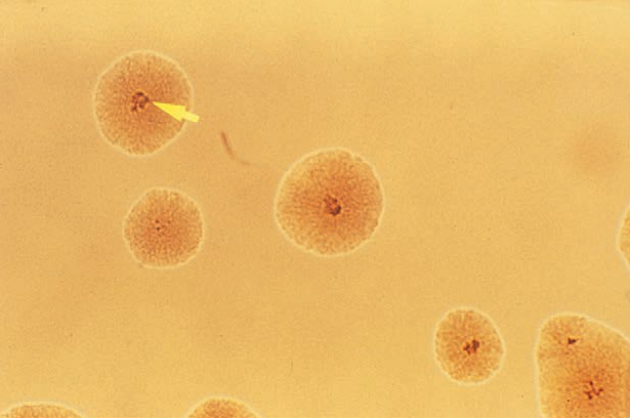 M. malmoense on Middlebrook 7H10 medium. The arrow indicates raised dark colony center.
M. malmoense on Middlebrook 7H10 medium. The arrow indicates raised dark colony center.
Growth rate is slow; optimal growth temperature is 37°C. Growth at 22°C may require as long as 7 weeks of incubation. Some strains may require longer incubation (up to 12 weeks) before colonies become visible which may lead it to underreporting.
Differential characteristics of M. malmoense are no accumulation of niacin, absence of nitrate reduction, ability to hydrolyze Tween 80, and production of a heat-stable catalase.
7. Mycobacterium marinum
M. marinum has been implicated in cutaneous infections when traumatized skin comes into contact with salt water or inadequately chlorinated fresh water containing the organism.
The typical presentation of a tender red or blue-red subcutaneous nodule, or swimming pool granuloma, usually occurs on the elbow, knee, toe, or finger.
Treatment includes simple observation of minor lesions, surgical excision, anti-TB drug therapy, and the use of single antimicrobial agents.
In in vitro AST, M. marinum is susceptible to rifampin and ethambutol, resistant to isoniazid and pyrazinamide, and partially resistant or intermediate to streptomycin.
Cells of M. marinum are long rods with cross barring. Colonies of this slowly growing organism are smooth to rough and wrinkled on inspissated egg medium but may be smooth when grown on Middlebrook 7H10 or 7H11 agar.
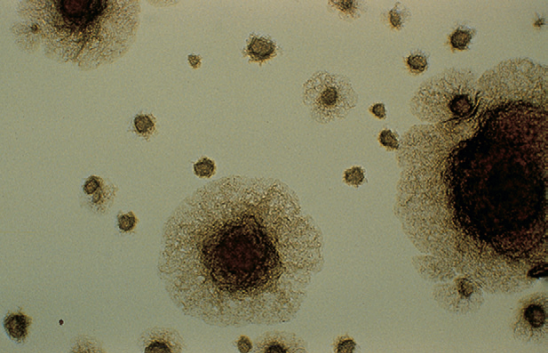 M. marinum on Middlebrook 7H10 medium producing rough colonies.
M. marinum on Middlebrook 7H10 medium producing rough colonies.
M. marinum is photochromogenic; young colonies grown in the dark may be nonpigmented or buff, whereas colonies grown in or exposed to light develop a deep yellow color. Growth is optimum at incubation temperatures of 28°C to 32°C.
The lower incubation temperatures, along with photochromogenicity, are clues to the identification of M. marinum.
Some strains of M. marinum produce niacin; however, none reduces nitrate or produces heat-stable catalase. The organisms hydrolyze Tween 80 and produce urease and pyrazinamidase.
8. Mycobacterium scrofulaceum
The most common form of disease associated with M. scrofulaceum is cervical lymphadenitis in children. The infection manifests in one or more enlarged nodes, often adjacent to the mandible and high in the neck, with little or no pain. Patients are usually treated by surgical incision and drainage; anti-TB drugs are generally not necessary. Pulmonary infections caused by M. scrofulaceum have been reported.
M. scrofulaceum is resistant to isoniazid, streptomycin, ethambutol, and p-aminosalicylic acid when tested in vitro.
Microscopically, M. scrofulaceum is a uniformly stained, acid-fast, medium to long rod.
The organism grows slowly (4 to 6 weeks) at incubation temperatures ranging from 25°C to 37°C.
Colonies are smooth with dense centers and pigmentation from light yellow to deep orange. The organism is scotochromogenic.
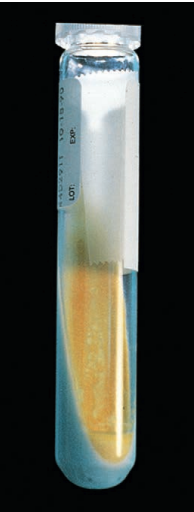 M. scrofulaceum on Löwenstein Jensen medium.
M. scrofulaceum on Löwenstein Jensen medium.
Members of this species do not hydrolyze Tween 80 or reduce nitrate, but do produce urease and are high catalase producers.
These characteristics aid in differentiating this organism from other slow-growing scotochromogens, including certain strains of MAC, M. gordonae, and M. szulgai.
9. Mycobacterium simiae
Infrequent cases of human infection from M. simiae manifest as pulmonary disease in patients with preexisting lung damage. Many isolates are resistant to most anti-TB drugs.
Cells of M. simiae appear as short coccobacilli. When they are grown on inspissated egg medium at 37°C, smooth colonies appear in 10 to 21 days.
Colonies on Middlebrook 7H10 agar are thin, transparent or tiny, and filamentous. The species is usually photochromogenic (yellow pigment) may require prolonged incubation, whereas some strains may fail to produce pigment on exposure to light.
Differential biochemical characteristics are the accumulation of niacin, negative nitrate reduction, and high-level, heat-stable catalase. M. simiae is one of the very few NTM that produces niacin, making it possible to confuse the identification with M. tuberculosis if pigment production under light is not observed.
10. Mycobacterium szulgai
With M. szulgai, the most common manifestation is pulmonary disease similar to TB.
Extrapulmonary infections, including lymphadenitis and bursitis, also have been reported. Isolation of this organism is almost always considered clinically significant.
On microscopic examination of an acid-fast–stained smear, cells of M. szulgai are medium to long rods, with some cross barring. When the organism is cultured on egg-based medium at 37°C, smooth and rough colonies are observed.
At 37°C, yellow to orange pigment develops in the absence of light and intensifies with exposure to light. Colonies grown at 22°C are nonpigmented or buff in the absence of light and develop yellow to orange pigment with light exposure.
Characteristics that differentiate M. szulgai from other slow-growing mycobacteria are slow hydrolysis of Tween 80, positive nitrate reduction, and inability to grow in the presence of 5% sodium chloride.
11. Mycobacterium ulcerans
The disease manifests as a painless nodule under the skin after previous trauma. A shallow ulcer develops that may be quite severe. Patients rarely develop fever or systemic symptoms.
The acid-fast cells of M. ulcerans are moderately long, without beading or crossbanding. Optimal growth temperature is 30°C to 33°C, with little growth at 25°C and usually none at 37°C. The organism grows slowly, often requiring 6 to 12 weeks of incubation before colonies are evident.
Colonies are smooth or rough and nonpigmented or lightly buff, and they do not develop pigment with exposure to light.
M. ulcerans produces a heat-stable catalase but is inert in most other biochemical tests.
12. Mycobacterium xenopi
M. xenopi infection are mostly slowly progressive pulmonary infections. Preexistent lung disease, alcoholism, malignancy, and diabetes mellitus are some of the conditions associated with M. xenopi infections. The pulmonary infections present a clinical picture similar to that seen in patients with M. tuberculosis, M. kansasii, or MAC infection. Disseminated and extrapulmonary infections have been reported.
Strains of M. xenopi are susceptible to the quinolones (ciprofloxacin, ofloxacin); some isolates are susceptible to vancomycin, erythromycin, or cefuroxime.
On acid-fast–stained smears, M. xenopi are long filamentous rods. Colonies of this slow-growing mycobacterium on Middlebrook 7H10 agar are small, with dense centers and filamentous edges.
Microscopic observation (x10) of colonies growing on cornmeal-glycerol agar reveals distinctive round colonies with branching and filamentous extensions; and aerial hyphae in rough colonies.
Furthermore, young colonies grown on cornmeal agar have a bird’s nest appearance, with sticklike projections. Optimal growth to is 42°C; and slow grow at 37°C and fails to grow at 25°C.
M. xenopi colonies are bright yellow in the absence of light and when exposed to light.
Distinctive characteristics, in addition to optimal growth at 42°C and yellow scotochromogenic pigment, are negative reactions for niacin accumulation, nitrate reduction, and positive reactions for the production of heat-stable catalase, arylsulfatase, and pyrazinamidase.
RAPIDLY GROWING SPECIES
1. Mycobacterium chelonae–Mycobacterium abscessus Group
The three most important rapidly growing mycobacteria causing human infections are M. abscessus subsp. abscessus, M. chelonae, and M. fortuitum.
M. chelonae is the species of rapidly growing mycobacteria most likely isolated from disseminated cutaneous infections in immunocompromised patients. Both M. fortuitum and M. chelonae have been associated with a variety of infections of the skin, lungs, bone, central nervous system, and prosthetic heart valves.
M. chelonae exhibits more resistance to antimicrobial agents than M. fortuitum but sometimes is susceptible to amikacin and a sulfonamide.
Approx. 80% of the cases of pulmonary disease caused by rapidly growing mycobacteria are caused by M. abscessus. It has also been seen in patients with cystic fibrosis (CF).
Microscopically, young cultures of M. chelonae are strongly acid-fast, with pleomorphism ranging from long, tapered to short, thick rods. This rapidly growing mycobacterium produces rough or smooth, nonpigmented to buff colonies within 3 to 5 days of incubation at 37°C.
A positive 3-day arylsulfatase test, no reduction of nitrate, and growth on MacConkey agar without crystal violet are characteristics that help differentiate M. chelonae and M. abscessus from other nonchromogenic, rapidly growing mycobacteria.
2. Mycobacterium fortuitum Group
Historically, the M. fortuitum group has been implicated in infections of the skin and soft tissues, including localized infections and abscesses at the site of puncture wounds. Among others, infections associated with long-term use of IV and peritoneal catheters, injection sites, and surgical wounds following mammoplasty and cardiac bypass procedures have been reported.
After 3 to 5 days of incubation at 37°C, colonies of M. fortuitum appear rough or smooth and nonpigmented, creamy white, or buff.
Microscopic examination of growth on cornmeal-glycerol and Middlebrook 7H11 agars after 1 to 2 days of incubation reveals colonies with branching filamentous extensions and rough colonies with short aerial hyphae.
Microscopically, cells are pleomorphic, ranging from long and tapered to short, thick rods. Cells from most cultures, especially older ones, tend to decolorize and appear partially acid-fast with any of the acid-fast staining techniques.
Additional characteristics that distinguish M. fortuitum from other rapidly growing mycobacteria are the positive 3-day arylsulfatase test and reduction of nitrate.
3. Mycobacterium smegmatis Group
The M. smegmatis group contains two species, M. smegmatis and M. goodie.
M. smegmatis has been implicated in rare cases of pulmonary, skin, soft tissue, bone infections, and granuloma in the soft tissue
Microscopically, on acid-fast stain, cells are long and tapered or short rods with irregular acid fastness. Occasionally rods are curved with branching or Y-shaped forms; swollen, with deeper staining, beaded, or ovoid forms are sometimes seen.
Colonies appearing on egg medium after 2 to 4 days are usually rough, wrinkled, or coarsely folded; smooth, glistening, butyrous colonies may also be seen.
Colonies on Middlebrook 7H10 agar are heaped and smooth or rough with dense centers. Pigmentation is rare or late; colonies appear nonpigmented, creamy white, or buff to pink in older cultures.
In addition to the rapid growth rate and nonpigmented rough colonies, characteristics valuable in the identification of this organism are its negative arylsulfatase reaction, positive iron uptake, ability to reduce nitrate, and growth in the presence of 5% NaCl and on MacConkey agar without crystal violet.
MYCOBACTERIUM LEPRAE
Mycobacterium leprae is the causative agent of Hansen disease (leprosy), an infection of the skin, mucous membranes, and peripheral nerves. The two major forms of the disease are tuberculoid leprosy and lepromatous leprosy.
Symptoms of tuberculoid leprosy include skin lesions and nerve involvement that can produce areas with loss of sensation. The optimal growth to for M. leprae is approx. 30°C, and because the patient mounts an adequate immune response, the bacteria tend to remain in the extremities.
Conversely, patients with lepromatous leprosy do not produce an effective CMI response. The disease is slowly progressive, and if untreated, life-threatening.
It is characterized by skin lesions and progressive, symmetric nerve damage. Lesions of the mucous membranes of the nose may lead to destruction of the cartilaginous septum, resulting in nasal and facial deformities.
Current therapy usually consists of a combination of diaminodiphenylsulfone (dapsone), clofazimine, and rifampin.
Laboratory diagnosis of Hansen disease depends on the microscopic demonstration of AFB that cannot be cultured from skin biopsy specimens. M. leprae has not been grown on artificial media.
In patients with tuberculoid leprosy, organisms are extremely rare and may not be detected in skin scrapings or biopsy specimens. However, AFB are usually abundant in samples from patients with lepromatous leprosy.
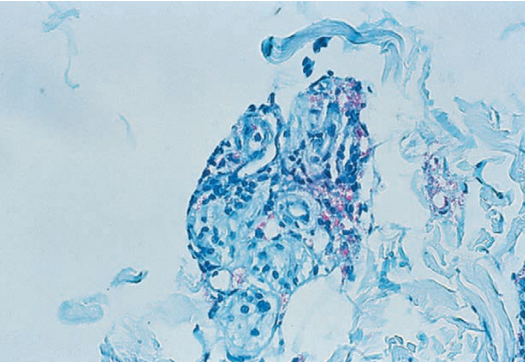 M. leprae from a skin biopsy from a patient with lepromatous leprosy
M. leprae from a skin biopsy from a patient with lepromatous leprosy
M. leprae is a rod-shaped bacterium, usually 1 to 7 µm long and 0.3 to 0.5 µm wide. After examination of the entire smear, the number of organisms present per oil immersion field (×1000) is reported as the bacteriologic index (BI). The number of solid-staining cells per 100 total bacilli examined is reported as the morphologic index (MI). Solid-staining cells are those with dense, uniform staining of the entire bacillus, with even sides and rounded ends, in which the length of the bacillus is at least five times the width of the bacillus.
The BI and MI aid the clinician in determining progression of the disease.
The definitive laboratory diagnosis is the development of disease in laboratory mice following inoculation of patient biopsy material to the mouse footpad.
END