Curriculum
SPECIALIST IN MICROBIOLOGY
MICROBIOLOGY LABORATORY PROCEDURES
0/4LAB. IDENTIFICATION OF SIGNIFICANT ISOLATES
0/14-
STAPHYLOCOCCACEAE
-
STREPTOCOCCACEAE AND OTHER RELATED ORGANISMS
-
ENTEROBACTERIACEAE
-
NEISSERIACEAE AND MORAXELLACEAE
-
HAEMOPHILUS AND OTHER FASTIDIOUS GRAM-NEGATIVE SPECIES
-
OTHER FASTIDIOUS GRAM-NEGATIVE BACILLI, BORDETELLA AND LEGIONELLA SPP.
-
VIBRIO, AEROMONAS, PLESIOMONAS, AND CAMPYLOBACTER SPECIES
-
NONFERMENTING GRAM-NEGATIVE BACILLI
-
ANAEROBES OF CLINICAL IMPORTANCE
-
THE SPIROCHETES
-
CHLAMYDIA, RICKETTSIA, AND SIMILAR ORGANISMS
-
MYCOPLASMA AND UREAPLASMA
-
MTB AND NTM LESSON 1
-
MTB AND NTM LESSON 2 - LABORATORY DIAGNOSIS
MEDICALLY SIGNIFICANT FUNGI
0/3DIAGNOSTIC PARASITOLOGY
0/5MYCOPLASMA AND UREAPLASMA
INTRODUCTION
This group of organisms once thought to be viruses because of their size. Mycoplasmas are the smallest self-replicating organisms in nature. Mycoplasma and Ureaplasma are the two genera in the family Mycoplasmataceae, class Mollicutes
The following species are the most significant human pathogens:
• Mycoplasma pneumoniae, which causes respiratory disease
• Mycoplasma hominis, associated with urogenital tract disease
• Ureaplasma urealyticum, associated with urogenital tract disease
1. General Characteristics
Mycoplasmas are pleomorphic organisms that do not possess a cell wall, which makes them resistant to cell wall– active antibiotics such as the penicillins and cephalosporins.
But they are not classified as L-forms, which are bacteria that have temporarily lost their cell wall as a result of environmental conditions; however, they are permanently lacking a cell wall.
They range in size from coccoid forms to tapered rods.
Mollicutes are generally slow-growing, highly fastidious, facultative anaerobes requiring complex media containing cholesterol and fatty acids for growth; important exceptions include aerobic M. pneumoniae and the more rapidly growing Mycoplasma hominis.
The mollicutes produce small colonies. Mycoplasma spp. often grow embedded beneath the surface of solid media; therefore, transferring colonies with a loop is ineffective.
On solid media, some species (e.g., M. hominis) form colonies with slightly raised centers giving the classic fried egg appearance.
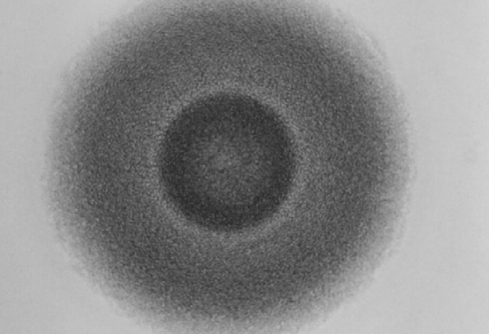 Typical large Mycoplasma colony showing fried egg appearance.
Typical large Mycoplasma colony showing fried egg appearance.
The mycoplasmas adhere to the epithelium of mucosal surfaces in the respiratory and urogenital tracts and are not eliminated by mucous secretions or urine flow.
Transmission of mycoplasmas and ureaplasmas in humans can occur via direct sexual contact, from mother to child during delivery or in utero, and by respiratory secretions or fomites in cases of M. pneumoniae infections.
2. Clinical Infections
· Mycoplasma pneumoniae
Mycoplasma pneumoniae may cause bronchitis, pharyngitis, known as primary atypical pneumonia, or walking pneumonia. Symptoms resemble those caused by Chlamydophila pneumoniae.
The disease differs from the typical pneumonia caused by Streptococcus pneumoniae in that it is milder, has a higher incidence in young adults, and is not seasonal.
Mycoplasma pneumoniae is not a normal biota; therefore, its isolation is always significant.
Transmission is through aerosol droplet by coughing. The most common presentation is tracheobronchitis; however, some patients demonstrate apparent pneumonia.
The incubation period is 2 to 3 weeks, and early symptoms consist of headache, low-grade fever, malaise, and anorexia. Sore throat, dry cough, and earache are accompanying symptoms.
Extrapulmonary complications, including cardiovascular, CNS, dermatologic, and GI problems, are rare occurrences.
M. pneumoniae is not associated with infections of the urogenital tract.
· Mycoplasma hominis and Ureaplasma Species
Although the mollicutes do not cause vaginitis, both M. hominis and U. urealyticum are associated with infections of the urogenital tract.
They are, however, frequently isolated from asymptomatic sexually active individuals, and the rate of colonization is directly related to the number of sexual partners. That makes interpretation of a positive culture difficult.
Mycoplasma hominis is found in the lower GUT of 50% of healthy adults and is not associated with nongonococcal urethritis (NGU).
The organism may, however, invade the upper genitourinary tract and cause salpingitis, pyelonephritis, pelvic inflammatory disease (PID), or postpartum fevers.
Ureaplasma parvum (U. urealyticum biovar 1) and U. urealyticum (U. urealyticum biovar 2) do not cause disease in the female lower genital tract but have been associated with 10% of cases of NGU in men.
Ureaplasma urealyticum is a common organism isolated from tracheal aspirates of neonates with respiratory disease even after caesarian delivery indicating that infection occurred in utero and not from the birth canal.
Both M. hominis and Ureaplasma spp. have been recovered from the CSF of preterm and low-birthweight babies.
In particular, U. parvum has been linked to respiratory distress in neonates.
3. Laboratory Diagnosis
Because recovery from culture is difficult, isolation of M. pneumoniae from respiratory sites is infrequently attempted. Growth may take several weeks, and technical expertise is necessary.
M. hominis is the only species that will grow on sheep blood and chocolate agars.
Diagnosis of M. pneumoniae infection is established serologically, with acute and convalescent sera collected 2 to 3 weeks apart to demonstrate a fourfold rise in titer.
Specimen Collection and Transport
Specimens for mycoplasmal culture include blood, sputum, synovial fluid, CSF, amniotic fluid, and urine; aspirates and nasopharyngeal, cervical, and vaginal swabs as well as tissue samples.
Because of the lack of a cell wall, all mycoplasmas are extremely sensitive to drying and heat.
Ideally, specimens should be inoculated at bedside. If this is not possible, specimens should be delivered immediately to the Lab in a transport medium, such as SP4 or Shepard’s 10B broth or 2SP, which are designed for the mycoplasma.
Cotton-tipped swabs and wooden shafts should be avoided because of possible inhibitory effects. Recommended swabs are made of Dacron polyester or calcium alginate with aluminum or plastic shafts and that swabs be removed when the sample is placed in a transport medium.
On arrival in the laboratory, the specimens should be frozen at −70° C if plating within 24 hours is not possible.
· Direct Examination
Because they lack a cell wall, the mollicutes will not be visible by Gram staining.
A DNA fluorescent stain (e.g., acridine orange) can be used, but this is not specific for the mollicutes.
· Culture
Media.
No single medium is suitable for all species isolated from humans. Penicillin can be added to minimize bacterial contamination. SP4 broth and agar are ideal for M. pneumoniae and M. hominis.
M. pneumoniae and M. genitalium require glucose (for energy), M. hominis requires arginine, and Ureaplasma spp. require urea.
Ureaplasma spp. also require media to have a pH near 6.0 (Shepherd’s 10B arginine broth).
It is difficult to maintain Ureaplasma spp. in culture because death occurs rapidly when the urea is depleted, and the bacteria are sensitive to changes in pH because of urea utilization.
Because mycoplasmas do not produce turbidity in broth media, a pH indicator such as phenol red should be added to detect growth.
A8 agar can be used as a solid medium to recover M. hominis and Ureaplasma spp.
Recovery of mycoplasma from blood can be performed by placing uncoagulated blood into mycoplasmal broth media. A ratio of 1:10 (blood to broth) and 10 mL of blood for adults is recommended.
Fluids should be centrifuged and the pellet resuspended in a small volume broth up to 10−3 for media inoculation. This helps minimize the inhibitors that may be present in the specimen.
· Isolation and Identification
Once inoculated, broth media should be placed at 37° C under atmospheric conditions, whereas solid agar media may be incubated in an environment of room air enhanced with 5% to 10% CO2, or in an anaerobic atmosphere of 95% N2 with 5% CO2. Incubation in a candle jar is adequate.
M. hominis and Ureaplasma spp. colonies may appear within 2 to 4 days, whereas M. pneumoniae may take 21 days or longer.
Mycoplasma-like colonies are stained with the Dienes or methylene blue stain.
Staining is performed by placing a small block of the agar on a glass slide, covering the colony with the stain, adding a coverslip, and examining the agar under low power.
M. hominis has a typical fried egg appearance, with the periphery staining a light blue and the center dark blue.
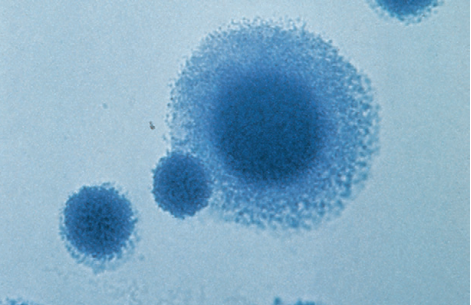 Dienes stain of Mycoplasma spp. Colonies. Note, typical fried egg appearance.
Dienes stain of Mycoplasma spp. Colonies. Note, typical fried egg appearance.
Although not conclusive, growth rate, body site recovered from, and colony appearance can aid in the identification of mycoplasma.
Glucose utilization in SP4 broth will cause an acid shift producing a yellow color, whereas arginine metabolism will produce a rise in pH, changing the indicator to a deeper red color. In 10B broth, urea or arginine utilization will increase the pH, changing the pH indicator from orange to deep red.
· A slow growing mycoplasma from a respiratory specimen producing a yellow color in SP4 broth is likely M. pneumoniae.
· Production of an alkaline reaction in 10B broth after overnight incubation of a urogenital specimen is suggestive of U. urealyticum, whereas
· An alkaline shift in media with arginine within 24 to 72 hours is likely M. hominis.
Antimicrobial Susceptibility
Because they lack a cell wall, the mollicutes are resistant to the β-lactams—penicillins and cephalosporins—as well as sulfonamides, trimethoprim, and rifampin.
M. pneumoniae has remained susceptible to the tetracyclines, newer fluoroquinolones, and the macrolides (e.g., erythromycin).
M. hominis, which is more resistant than M. pneumoniae, is resistant to erythromycin but susceptible to clindamycin and lincomycin, whereas U. urealyticum is resistant to clindamycin and lincomycin and sensitive to erythromycin.
Thank you