Curriculum
iMLS-MICROBIOLOGY
MICROBIOLOGY LABORATORY PROCEDURES
0/8LAB. IDENTIFICATION OF SIGNIFICANT ISOLATES
0/36-
STAPHYLOCOCCACEAE
-
RECORDED LECTURE - STAPHYLOCOCCACEAE
-
STREPTOCOCCACEAE AND OTHER RELATED ORGANISMS
-
RECORDED LECTURE - STREPTOCOCCUS PART II
-
ENTEROBACTERIACEAE
-
RECORDED LECTURE - ENTEROBACTERIACEAE PART I
-
RECORDED LECTURE - ENTEROBACTERIACEAE PART II
-
NEISSERIACEAE AND MORAXELLACEAE
-
RECORDED LECTURE - NEISSERIACEAE PART I
-
RECORDED LECTURE- NEISSERIACEAE PART II
-
HAEMOPHILUS AND OTHER FASTIDIOUS GRAM-NEGATIVE SPECIES
-
OTHER FASTIDIOUS GRAM-NEGATIVE BACILLI, BORDETELLA AND LEGIONELLA SPP.
-
RECORDED LECTURE - OTHER FASTIDIUS GRAM NEGATIVE BACILLI
-
VIBRIO, AEROMONAS, PLESIOMONAS, AND CAMPYLOBACTER SPECIES
-
RECORDED LECTURE - VIBRIONACEAE PART I
-
RECORDED LECTURE - VIBRIONACEAE PART II
-
NONFERMENTING GRAM-NEGATIVE BACILLI
-
RECORDED LECTURE - NON-FERMENTERS PART I
-
RECORDED LECTURE - NONFERMENTERS PART I
-
ANAEROBES OF CLINICAL IMPORTANCE
-
RECORDED LECTURE- ANAEROBES PART I
-
RECORDED LECTURE- ANAEROBES PART II
-
THE SPIROCHETES
-
RECORDED LECTURE- THE SPIROCHETES
-
THE SPIROCHETES PRACTICE QUESTIONS
-
CHLAMYDIA, RICKETTSIA, AND SIMILAR ORGANISMS
-
RECORDED LECTURE - CHLAMYDIA AND RICKETTSIA
-
MYCOPLASMA AND UREAPLASMA
-
RECORDED LECTURE-MYCOPLASMA AND UREAPLASMA
-
MTB AND NTM LESSON 1
-
RECORDED- LECTURE MTB PARTI
-
RECORDED LECTURE - NTM
-
MTB AND NTM LESSON 2 - LABORATORY DIAGNOSIS
-
RECORDED LECTURE - MTB LAB DIAGNOSIS
-
RECORDED LECTURE - MTB LAB IDENTIFICATION
-
MTB & NTM PRACTICE QUESTIONS
Medically Significant Fungi
0/7Diagnostic Parasitology
0/12MTB AND NTM LESSON 2 – LABORATORY DIAGNOSIS
PART II. Isolation and Identification of the Mycobacteria
1. Introduction
Rate of growth, colony morphology, pigmentation, nutritional requirements, optimal incubation temperature, and biochemical test results are traditional features used to differentiate species within the genus Mycobacterium.
More rapid techniques include broth-based culture systems (that monitor cultures continuously during the incubation period).
Species-specific nucleic acid probes (PCR) and HPLC for rapid identification of culture isolates to the species level.
2. Specimen Collection
Mycobacteria may be recovered from respiratory specimens, urine, feces, blood, CSF, tissue biopsies, and aspirations of any tissue or organ.
If immediate transport to the Lab is not possible, the specimen may be refrigerated overnight. The recommended container is a sterile, wide-mouth cup with a tightly fitted lid. Because of small sample volume, the use of swabs is discouraged.
The spectrum of illness caused by Mycobacterium spp. is so broad that almost any site can yield an acceptable specimen.
· Sputum and Other Respiratory Secretions
Sputum and bronchial aspirates are the most common specimens submitted to recover MTB and NTM. An early-morning specimen should be collected on three consecutive days. Pooled specimens are unacceptable because of increased contamination.
A volume of 5 to 10 mL of sputum produced by a deep cough of expectorated sputum or aerosol induced should be used. When sputum is not obtainable, bronchoscopy may be performed, at which time samples such as bronchial washing, bronchoalveolar lavage (BAL), or transbronchial biopsy specimens are obtained. Brushings appear to be better than washing or biopsy.
· Gastric Aspirates and Washings
Gastric aspirates are used to recover mycobacteria that may have been swallowed during the night. This is used only for patients who do not produce sputum by aerosol induction, for children younger than 3 years, and for non-ambulatory individuals.
Gastric lavage has been reported to be better than BAL for the detection of mycobacteria in children. Gastric lavage may be alternative only for those unable to expectorate sputum and for whom BAL might be contraindicated.
Gastric aspirates should be obtained in the morning after an overnight fast. Three specimens should be collected within 3 days. Sterile water, 30 to 60 mL, is instilled orally or via nasogastric tube aspiration. Prolonged exposure to gastric acid kills mycobacteria and diminishes culture yield. Specimen should be neutralized with sodium carbonate or another buffer to pH 7.0 as soon as possible after specimen collection.
· Urine
First morning midstream specimen is preferred. Minimum 15 mL is collected in a sterile container. A specimen may be collected through an indwelling catheter with a sterile needle and syringe.
The specimens should be refrigerated during the interval between collection and processing; specimens should be processed promptly. As a general rule, pooled specimens collected over 12 to 24 hours are not recommended. Such specimens are more subject to contamination and may contain fewer viable tubercle bacilli.
· Stool
Stool specimens can be useful in AIDS patients, who may be at risk for developing disseminated mycobacterial disease resulting from MAC. The number of organisms found in the bowel in these patients is quite high. Stool specimens should be collected in clean containers without any preservative and sent directly to the lab for processing. If processing within a few hours is not possible, the specimen should be frozen at −20°C until processed. Stoll culture for mycobacteria is only warranted for AIDS.
· Blood, Tissue and Other Body Fluids
Mycobacteremia is rare, but is often seen in patients with AIDS. Most infections are caused by MAC.
Whenever possible, CSF specimens should be from large-volume spinal taps to increase diagnostic yield. Diagnosis of tuberculous meningitis is extremely difficult. Peritoneal (ascitic fluid) smears are also rarely positive for AFB. Culture of large volumes and inoculation of the specimen into mycobacterial liquid media can help maximize yield in dilute specimens.
When noninvasive techniques have failed to provide a diagnosis, surgical procedures may need to be considered. Specimens obtained from the lung, pericardium, lymph nodes, bones, joints, bowel, or liver may be appropriate.
The tissue or fluid should be collected aseptically and placed in a sterile container. If the tissue is not processed immediately, a small amount (10 to 15 mL) of sterile saline should be added to prevent dehydration. The amount of fluids recommended for culture varies—2 mL for CSF, 3 to 5 mL for exudates and pericardial and synovial fluids, and 10 to 15 mL for abdominal and chest fluids. Immediate processing of these samples is important.
Digestion and Decontamination of Specimens
Specimens from sterile body sites can simply be concentrated by centrifugation (if a large volume) and inoculated. However, specimens that may contain commensals should be decontaminated and then concentrated.
The purposes of the digestion-decontamination process are as follows:
(1) to liquefy the sample through digestion of the proteinaceous material; and
(2) to allow the chemical decontaminating agent to contact and kill the nonmycobacterial organisms.
The high lipid content in the cell wall of mycobacteria makes them somewhat less susceptible to the killing action of various chemicals. Specimens that contain mucus and require digestion and decontamination are sputum, gastric washing, BAL, bronchial washing, and transtracheal aspirate.
Voided urine, autopsy tissue, abdominal fluid, and any contaminated fluid require decontamination. Specimens from normally sterile sites, such as blood, CSF, synovial fluid, and biopsy tissue from deep organs, do not require decontamination. Sterility should be strictly maintained in collection and transport. Stool decontamination is especially difficult and may require repeated attempts.
· Decontamination and Digestion Agents
Sodium Hydroxide (2%, 3%, or 4%): It must be used with caution because it is only slightly less harmful to the mycobacteria than to the contaminating organisms.
N-Acetyl-L-cysteine: NALC is a liquefying agent that allow decontaminating agent (NaOH) to come into close contact with contaminating bacteria more readily.
Benzalkonium Chloride. Combined with trisodium phosphate (Z-TSP). TSP liquefies sputum rapidly but requires a long exposure time to decontaminate the specimen. Benzalkonium chloride shortens the exposure time and effectively destroys many contaminants, with little bactericidal effect on the tubercle bacilli.
Oxalic Acid. Oxalic acid, 5%, is used to decontaminate specimens contaminated with Pseudomonas aeruginosa, such as sputum specimens from patients with CF. Oxalic acid–treated specimens can be used with the broth-based culture systems.
Concentration Procedures
The specific gravity of the tubercle bacilli ranges from 0.79 to 1.07. Because of the low SG of the AFB, a low centrifugal force has a buoyant rather than a sedimenting effect. Excess mucus will worsen this phenomenon.
Treatment with mucolytic agents such as NALC splits mucoprotein, allowing greater sedimentation. Concentration centrifugation speeds must be at least 3000 × g to maximize recovery.
Staining for Acid-Fast Bacilli
When Gram-stained, Mycobacterium spp. stain faintly or not at all. Acid-fast smears are prepared directly from clinical specimens and from digested, decontaminated, and concentrated specimens. The conventional acid-fast staining methods, Ziehl-Neelsen and Kinyoun stains, use carbolfuchsin as the primary stain, acid-alcohol as a decolorizing agent, and a methylene blue counterstain.
The Ziehl-Neelsen staining involves the application of heat with the carbolfuchsin stain, whereas the Kinyoun acid-fast stain is a cold stain.
Slides are examined using a ×100 oil immersion objective on a light microscope for 15 minutes, viewing a minimum of 300 fields before a slide is called negative.
The auramine or auramine-rhodamine fluorochrome stains are more sensitive than the carbolfuchsin stains. In addition, smears can be screened at a lower magnification (×250 to ×400), thus allowing for more fields to be examined in a shorter time.
A fluorescence microscope equipped with an appropriate filter system is needed for the examination of a fluorochrome-stained smear. The smear is examined under a mercury vapor lamp with a strong blue-filtered light.
Positive stains reveal bright, yellow-orange bacilli against a dark background. Smears should be carefully examined with a minimum of 300 fields, and three horizontal sweeps of a smear that is 2 cm long and 1 cm wide should be performed.
A few rapidly growing mycobacteria may be acid-fast; they may not stain at all with fluorochrome stains, so suspected, smears should be stained with carbolfuchsin and a weaker decolorizing process used.
In the interpretation of a smear as positive for AFB, lab scientists must realize that organisms other than Mycobacterium might stain at least partially acid-fast (e.g., Nocardia spp., Legionella micdadei, and Rhodococcus spp.).
Culture Media and Isolation Methods
Mycobacteria are strictly aerobic and grow more slowly than most bacteria pathogenic for humans. The generation time of mycobacteria is longer than 12 hours; M. tuberculosis has the longest replication time, at 20 to 22 hours. The rapidly growing species generally form colonies in 2 to 3 days, whereas most pathogenic mycobacteria require 2 to 6 weeks of incubation. The growth of M. tuberculosis is enhanced by an atmosphere of 5% to 10% CO2, pH 6.5 – 6.8 and higher humidity.
M. genavense, does not grow on routine media and requires extended incubation (6 to 8 weeks), whereas M. leprae fails to grow on artificial media.
Media used to isolate mycobacteria are variations of three general types—egg-based media, serum albumin agar media, and liquid media. Within each general type, there are nonselective and selective formulations.
1. Egg-Based Media
The basic ingredients in an inspissated egg medium, such as Löwenstein–Jensen medium (most commonly used), Petragnani, and American Thoracic Society (ATS) media, are fresh whole eggs, potato flour, and glycerol, with slight variations in defined salts, milk, and potato flour. Each contains malachite green to suppress the growth of gram-positive bacteria. Selective media include Gruft modification of LJ and Mycobactosel, are used for contaminated specimens.
2. Agar-Based Media
Serum albumin agar media, such as Middlebrook 7H10 and 7H11 agars, are prepared salts, vitamins, cofactors, glycerol, malachite green, and agar combined with an enrichment consisting of oleic acid, bovine albumin, glucose, and beef catalase. Middlebrook 7H11 medium also contains 0.1% casein hydrolysate, which improves the recovery of isoniazid-resistant strains of M. tuberculosis.
The addition of antimicrobial agents to 7H10 or 7H11 makes the media selective. Mitchison’s selective 7H11 contains polymyxin B, amphotericin B, carbenicillin, and trimethoprim lactate. In contrast to opaque egg-based media, clear agar-based media can be examined using a dissecting microscope for early detection of growth and colony morphology and drug susceptibility tests may be performed without altering drug concentrations, which occurs with egg-based media.
Middlebrook 7H10 and 7H11 media are incubated in an atmosphere of 10% CO2 and 90% air; 99% of the positive cultures are detected in 3 to 4 weeks, earlier than for those detected on egg-based media.
3. Liquid Media
Mycobacterium spp. grow more rapidly in liquid medium, and it can be used for both primary isolation and subculturing. Middlebrook 7H9 broth and Dubos Tween albumin are nonselective liquid media used for subculturing stock strains, picking single colonies, and preparing inoculum for in vitro testing. Numerous automated systems are available that use liquid media including BACTEC 460TB system, and MGIT 960 system.
Other Culture Media for Recovery of Mycobacteria
A CHOC agar plate should be included in the primary isolation media for skin and other body surface specimens for the recovery of M. haemophilum, which requires ferric ammonium citrate or hemin for growth. The plate should be incubated at 30°Cfor recovery of this organism and for M. marinum.
Alternatively, hemin (X) strips or disks used for the identification of Haemophilus spp. can be placed on Middlebrook agar plates.
A biphasic media system for the detection and isolation of mycobacteria, Septi-Chek AFB consists of a bottle containing Middlebrook 7H9 broth with an atmosphere of 5% to 8% CO2, an enrichment consisting of growth-enhancing factors and antimicrobial agents, and a paddle with agar media. One side of the paddle is covered with nonselective Middlebrook 7H11 agar.
One of the two sections on the reverse side of the paddle contains a modified egg-based medium for differentiating M. tuberculosis from other mycobacteria, and the other side contains CHOC agar for the detection of contaminating bacteria.
The biphasic media system provides for rapid growth and identification and drug susceptibility testing, without the need for routine subculturing.
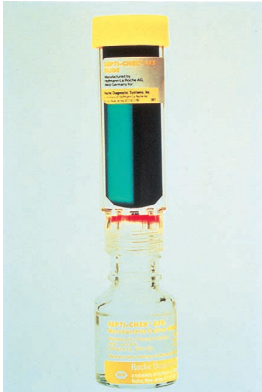 Septi-Chek biphasic media system for acid-fast bacilli
Septi-Chek biphasic media system for acid-fast bacilli
Isolator Lysis-Centrifugation System
Isolator is a blood collection system that contains saponin to liberate intracellular organisms. After treatment with the saponin, the sample is inoculated onto mycobacteria media plates or tubes. The system allows for higher yields and shorter recovery times for mycobacteria.
Laboratory Identification
Preliminary Identification of Mycobacteria
The first step is to confirm that the isolate recovered in broth or on solid media is an acid-fast organism by performing an acid-fast stain. Then, once the organisms are growing on solid media,characteristics such as colony morphology, growth rate, optimal growth temperature, and photoreactivity help speciate mycobacteria.
Colony Morphology. Colonies of mycobacteria are generally distinguished as having a smooth and soft or rough and friable appearance. Colonies of M. tuberculosis that are rough often exhibit a prominent patterned texture referred to as cording (curved strands of bacilli); this texture is the result of tight cohesion of the bacilli. Colonies of MAC may appear glossy whitish often occurring with smaller translucent colonies.
Growth Rate. Growth rate depends on the species, the media, incubation temperature, and initial inoculum size. The range in recovery time is wide, from 3 to 60 days. Mycobacteria are categorized as rapid growers, having visible growth in <7 days, or slow growers, producing colonies in >7 days.
Growth rate should be evaluated from the time of subculture, not the time of detection from the clinical sample. The inoculum should be sufficiently small to produce isolated colonies.
Temperature. The range at which a mycobacterial species can grow may be extremely narrow, especially at the time of initial incubation. M. marinum, M. ulcerans, and M. haemophilum grow best at 30°C to 32°C and poorly, if at all, at 35°C to 37°C. At the other extreme, M. xenopi grows best at 42°C.
Photoreactivity. Mycobacterium spp. are categorized into three groups. Species that produce carotene pigment on exposure to light are photochromogens. Color ranges from pale yellow to orange. Examples include; M. asiaticum, M. kansasii, M. marinum, M. simiae
Species that produce pigment in the light or the dark are scotochromogens e.g., M. gordonae, M. szulgai, M. scrofulaceum, M. xenopi.
Other species, such as M. tuberculosis, M. avium-intracellulare, M. bovis, M. celatum, M. gastri, M. genavense, M. haemophilum, M. malmoense, M. terrae complex and M. ulcerans are nonchromogenic or nonphotochromogenic. These colonies are a buff (tan) color.
Biochemical Identification
1. Niacin Accumulation
Most mycobacteria possess the enzyme that converts free niacin to niacin ribonucleotide. However, 95% of M. tuberculosis produce free niacin (nicotinic acid) because this species lacks the niacin-connecting enzyme.
Accumulation of niacin, detected as nicotinic acid, is the sensitive test for MTB. Nicotinic acid reacts with cyanogen bromide in the presence of an amine to form a yellow-pigmented compound.
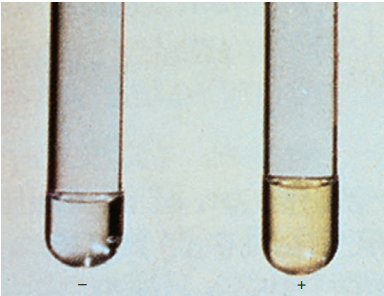 Niacin test.
Niacin test.
It is recommended that the test be done on egg agar cultures 3 to 4 weeks old and with at least 50 colonies. Tests that yield negative results may need to be repeated in several weeks. The test should not be performed on scotochromogenic or rapidly growing species because some may be positive, although this occurs rarely.
2. Nitrate Reduction
The reduction of nitrate to nitrite, is relatively uncommon among Mycobacterium spp., but a positive result may be seen in M. kansasii, M. szulgai, M. fortuitum, and M. tuberculosis.
Bacteria are incubated in 2 mL of sodium nitrate at 37°C for 2 hours. HCl 50%, sulfanilamide, and N-naphthylenediamine dihydrochloride are then added; if the bacteria reduce the nitrate to nitrite, a red color forms.
 Nitrate reduction test
Nitrate reduction test
When no color change develops, however, either no reaction has occurred or the reaction has gone beyond nitrite. The addition of zinc detects nitrate and results in a pink color change in a true-negative reaction.
The nitrate reduction test differentiates M. tuberculosis from the scotochromogens and MAC.
3. Catalase
Mycobacteria are catalase positive. Isolates that are catalase-positive after heating 68°C for 20 minutes have a heat-stable catalase.
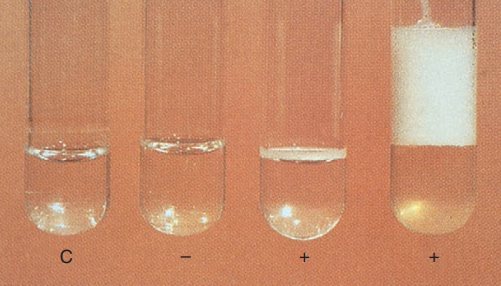 Catalase test
Catalase test
Most M. tuberculosis complex do not produce heat-stable catalase; exceptions are certain strains resistant to isoniazid. Other heat-stable, catalase-negative species include M. gastri, M. haemophilum, and M. marinum.
Semi-quantitation of catalase production is based on the addition of Tween 80 (a detergent) and hydrogen peroxide to a 2-week-old culture grown in an agar deep. The reaction is read after 5 minutes, and the resulting column of bubbles is measured as greater than or less than 45 mm.
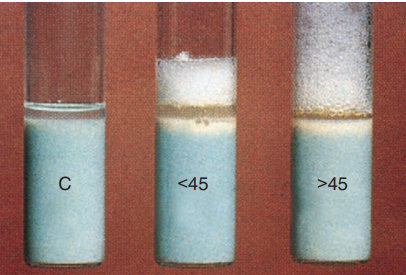 Semiquantitative catalase test.
Semiquantitative catalase test.
4. Hydrolysis of Tween 80.
Some mycobacteria possess a lipase that can split the detergent Tween 80 into oleic acid and polyoxyethylated sorbitol.
The pH indicator, neutral red, is initially bound to Tween 80 and has an amber color. After hydrolysis of Tween 80, neutral red can no longer bind, and it is released, causing a pink color to form. Results are recorded as positive after 24 hours, 5 days, or 10 days.
This test is helpful in distinguishing between scotochromogenic and nonphotochromogenic mycobacteria.
5. Iron Uptake
Some mycobacteria can convert ferric ammonium citrate to an iron oxide. After growth of the isolate appears on an egg-based medium slant, on the addition of 20% aqueous solution of ferric ammonium citrate, colonies appear rusty brown as a result of iron uptake.
 Iron uptake
Iron uptake
The test is most useful in distinguishing M. chelonae, which is negative, from other rapid growers, which are positive.
6. Arylsulfatase
Most Mycobacteria possess the enzyme arylsulfatase. This enzyme hydrolyzes the bond between the sulfate group and aromatic ring structure in Tripotassium phenolphthalein sulfate phenolphthalein is then liberated that causes a pH change in the presence of sodium bicarbonate, indicated by the formation of a pink color change.
The M. fortuitum complex, M. chelonae, M. xenopi, and M. triviale have rapid arylsulfatase activity that can be detected in 3 days. M. marinum and M. szulgai exhibit activity with 14 days of incubation.
7. Pyrazinamidase
Pyrazinamidase hydrolyzes pyrazinamide to pyrazinoic acid and ammonia in 4 days. Ferrous ammonium sulfate combines with pyrazinoic acid, producing a red pigment.
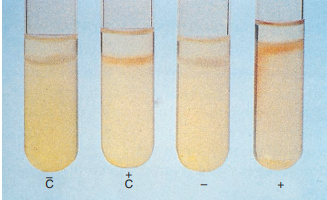 Pyrazinamidase test.
Pyrazinamidase test.
This reaction may be useful in distinguishing M. marinum from M. kansasii and M. bovis from M. tuberculosis.
8. Tellurite Reduction
Reduction of colorless potassium tellurite to black metallic tellurium in 3 to 4 days is a characteristic of MAC and thus is useful in distinguishing MAC from other nonchromogenic species.
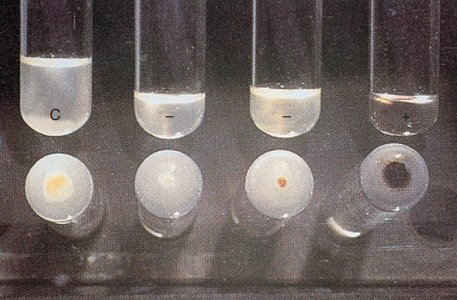 Test for tellurite reduction
Test for tellurite reduction
In addition, all rapid growers are able to reduce tellurite in 3 days.
9. Urease
Detection of urease activity can be used to distinguish M. scrofulaceum, urease-positive, from M. gordonae, urease-negative. A loopful of test organism is grown in 4 mL of urea broth at 37°C for 3 days. A pink to red color is indicative of a positive reaction.
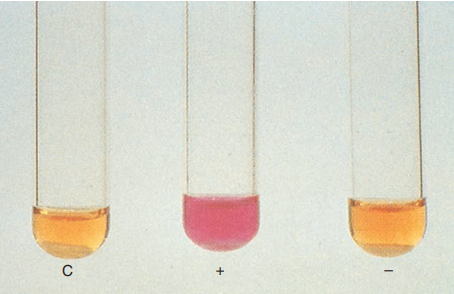 Urease test
Urease test
Inhibitory Tests
1. NAP
NAP (p-nitroacetylamino-β-hydroxypropiophenone) is a precursor in the synthesis of chloramphenicol. NAP selectively inhibits the M. tuberculosis complex.
An aliquot of the original BACTEC medium is transferred to the BACTEC-NAP vial, which contains 5 mg of NAP, growth is then closely monitored. A 20% increase in growth index is considered significant and rules out M. tuberculosis complex.
2. Thiophene-2-Carboxylic Acid Hydrazide
T2H distinguishes M. bovis from M. tuberculosis. M. bovis is susceptible to lower concentrations of T2H than MTB.
3. Sodium Chloride Tolerance
High salt conc. (5% NaCl) in egg-based media (e.g., LJ) inhibits the growth of most mycobacteria. M. flavescens, M. triviale, and most rapidly growing Mycobacterium spp. are exceptions that do grow in the presence of 5% NaCl.
4. Growth on MacConkey Agar
The Mycobacterium fortuitum-chelonae complex can grow on MacConkey agar without crystal violet (ordinary MAC), whereas most other mycobacteria cannot.
END